Rate Constant For Zero Order Reaction Is . Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. It is also known as the reaction rate. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate.
from www.tessshebaylo.com
Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. It is also known as the reaction rate. This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k.
Rate Constant Equation For Zero Order Tessshebaylo
Rate Constant For Zero Order Reaction Is The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k. It is also known as the reaction rate. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate.
From chem.libretexts.org
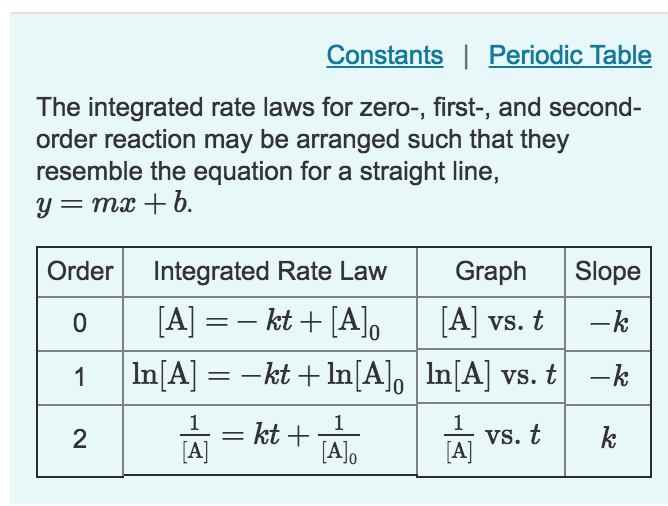
Chapter 14.4 Using Graphs to Determine Rate Laws, Rate Constants and Rate Constant For Zero Order Reaction Is If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k. This means that. Rate Constant For Zero Order Reaction Is.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Rate Constant For Zero Order Reaction Is Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. It is also known as the reaction rate. This means that the rate of the reaction is equal to the rate constant, k, of. Rate Constant For Zero Order Reaction Is.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Rate Constant For Zero Order Reaction Is Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k. This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. It is also known as the. Rate Constant For Zero Order Reaction Is.
From www.youtube.com
Zero Order Reactions Chemistry Class 12 IIT JEE Main + Advanced Rate Constant For Zero Order Reaction Is The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. It is also known as the reaction rate. Rate = k[a]0 = k (1) (1) r a t. Rate Constant For Zero Order Reaction Is.
From www.toppr.com
Derive the relationship between half life period and rate constant of a Rate Constant For Zero Order Reaction Is The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k. If it's zero order with respect to a, we can write that the rate of the reaction is. Rate Constant For Zero Order Reaction Is.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Rate Constant For Zero Order Reaction Is Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. This means that. Rate Constant For Zero Order Reaction Is.
From www.toppr.com
Derive an integrated rate equation for rate constant of a zero order Rate Constant For Zero Order Reaction Is This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. It is also known as the reaction rate. Rate = k[a]0 = k (1) (1) r a t e = k. Rate Constant For Zero Order Reaction Is.
From general.chemistrysteps.com
ZeroOrder Reactions Chemistry Steps Rate Constant For Zero Order Reaction Is This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. It is also known as the reaction rate. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates. Rate Constant For Zero Order Reaction Is.
From www.coursehero.com
[Solved] 2. Determine the units of the rate constants of (a) ZeroOrder Rate Constant For Zero Order Reaction Is It is also known as the reaction rate. This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. The rate constant is a proportionality factor in the rate law of chemical. Rate Constant For Zero Order Reaction Is.
From www.youtube.com
The unit of rate constant for a zero order reaction is `s^(1)`. YouTube Rate Constant For Zero Order Reaction Is Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. It is also known as the reaction rate. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. This means that the rate of the reaction is equal. Rate Constant For Zero Order Reaction Is.
From mungfali.com
The Rate Constant For Zero Order Reaction Is Youtube EF4 Rate Constant For Zero Order Reaction Is If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Rate = k[a]0 = k (1) (1) r a t e = k [ a]. Rate Constant For Zero Order Reaction Is.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Rate Constant For Zero Order Reaction Is Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical.. Rate Constant For Zero Order Reaction Is.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Rate Constant For Zero Order Reaction Is This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants. Rate Constant For Zero Order Reaction Is.
From www.toppr.com
The unit of rate constant for a zero order reaction is Rate Constant For Zero Order Reaction Is Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k. This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. Rate laws or. Rate Constant For Zero Order Reaction Is.
From saylordotorg.github.io
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant For Zero Order Reaction Is Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. It is also known as the reaction rate. This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. If it's zero order with respect to a, we can write that the rate of the. Rate Constant For Zero Order Reaction Is.
From schools.aglasem.com
CBSE Class 12 Chemistry Notes Chemical Rate Constant For Zero Order Reaction Is If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical. It is also. Rate Constant For Zero Order Reaction Is.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions Rate Constant For Zero Order Reaction Is If it's zero order with respect to a, we can write that the rate of the reaction is equal to the. Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k. This means that the rate of the reaction is equal to the rate constant, k, of that reaction,. It is also. Rate Constant For Zero Order Reaction Is.
From www.slideserve.com
PPT Summary of the of ZeroOrder, FirstOrder and Second Rate Constant For Zero Order Reaction Is Rate = k[a]0 = k (1) (1) r a t e = k [ a] 0 = k. The rate constant is a proportionality factor in the rate law of chemical kinetics that relates the molar concentration of reactants to reaction rate. Rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical.. Rate Constant For Zero Order Reaction Is.